A handful of hours before I started this essay, something really exciting happened. The FDA approved a medication called Cobenfy, a combination of xanomeline and trospium chloride, for the treatment of schizophrenia.
What’s exciting is that my decision to use the term “antidopaminergic” instead of “antipsychotic” has now been vindicated.
What’s exciting is that this one has a novel mechanism of action. There is no direct antidopaminergic activity. Xanomeline is an agonist at the M1 and M4 receptors. It’s the first novel therapeutic for schizophrenia in something like 70 years!
Er, probably? People who really know their psychopharm might remember that clozapine is also an agonist at M4 and its major metabolite norclozapine is an agonist at M1!1 I’ve suspected that this might be more than just trivia, but this is still just a pet theory.
I’m going to talk about the details a little bit, but if you want a really — and I mean really — thorough overview of the muscarinic receptors as therapeutic targets in schizophrenia I would recommend you read this overview in the Sept ‘22 edition of the AJP from Paul et al..
If you just want to skip to my breakdown of the study below, here’s a tl;dr: Xanomeline is an agonist at M1 and M4. Trospium is a antimuscarinic that does not cross the blood brain barrier, and is included to block xanomeline’s action outside of the CNS.
A Little Pharmacology
Muscarinic agonists are not a common therapeutic class in medicine. There’s bethanechol, a peripherally acting agonist to prevent post-op ileus and urinary retention, a couple of compounds used as topicals to manage glaucoma, and methacholine, which we use to induce asthma attacks in people… so we can diagnose them with asthma. Medicine, what a field!
You may have noticed that I didn’t specify which muscarinic receptors these drugs activate, that’s because it’s all of them. M1 through M5.
See, even though we’ve characterized five different muscarinic receptors, their binding sites are so similar that it is really, really hard to design a molecule that only binds to a subset of them. For whatever reason, it’s been much easier to find selective antagonists (or inverse agonists). I’m guessing that this is because antagonists just have to block the binding site, not bind in just the right way to activate the receptor; it’s very easy to imagine how you might block a keyhole, but much harder to design an effective lockpick.2
So, xanomeline being a selective muscarinic agonist at M1 and M4 makes it even more unique. Turns out that xanomeline — just like every other muscarinic agonist — actually has high affinity for all of the muscarinic receptors, but is not equally potent at each.
First, xanomeline binds allosterically3 at both M1 and M4 in addition to binding the orthosteric (typical) binding site for these receptors. It apparently sticks quite tightly to this allosteric site and — at least for M1 — seems to directly and persistently activate the receptor when bound4. It also appears to bind quite tightly to the M5 receptor, thought it instead acts an antagonist.5
Xanomeline also exhibits functional selectivity,6 which means that its binding results in activation of alternative G-protein pathways relative to the endogenous ligand. For example, a receptor that was formerly G(q) dominant might become more G(i) dominant when bound by a functionally selective ligand.
A Little History
Xanomeline was originally developed in the 1990’s as part of an effort to treat the cognitive symptoms of Alzheimer’s Disease. It was one of many muscarinic agonists designed to restore the cholinergic tone caused by a loss of neurons that project to the hippocampus. Most were intolerable due to their side-effects and didn’t have much of an effect on cognition. Xanomeline also had side-effects, only did an OK job at improving cognition, but it significantly reduced — and even appeared to prevent — behavioral and psychotic symptoms in demented patients.
You can see where this is going.
In 2008 Shekhar et al. published a small (N = 20) 4-week, randomized, placebo controlled trial of xanomeline (no trospium yet!) in moderately ill schizophrenics. Target dose was 225mg qDay.
This study showed a statistically significant and clinically meaningful improvement in total PANSS scores (μ improvement = 24 points), though just barely (p = 0.039). They also showed statistically significant improvements on a few cognitive tasks: digit span, story recall, list learning, and delayed memory. However, these were only 4 of 18 separate cognitive measures and the p-values were not particularly robust; the only exception was for story recall (p < 0.01). The rest were just a bit under 0.05.
As you might expect with a muscarinic agonist, there were a lot of gastrointestinal side-effects: nausea, vomiting, diarrhea, and so on. That said, the authors report that these were generally mild and no participant withdrew from the study for these reasons.
In 2016 Breier et al. did the first Phase I trial in which xanomeline 75mg TID was co-administered with trospium 20mg BID and found that it reduced the rate of xanomeline-induced side-effects by about 50%. A second Phase I trial published in 2019 simplified the dosing regimen with BID trospium, and found similar success. KarXT was off to the races.
Ok Ok Let’s Talk About The Paper
Efficacy and Safety of Xanomeline-Trospium Chloride in Schizophrenia A Randomized Clinical Trial by Kaul et al. published the August edition of JAMA Psychiatry.
From here on out, I’m just going to refer to the drug as “xanomeline” instead of xanomeline/trospium all the time. Combination drugs are the one time I’m really tempted to use brand names, but I refuse to let Big Pharma win. Also KarXT is a way cooler name than Cobenfy.
Methods
This was a Phase 3, multicenter, randomized, double-blind, placebo controlled trial carried out in the US and Ukraine7 between April 1, 2021 to December 7, 2022.
Inclusion Criteria
This is a summary, you can find full inclusion/exclusion criteria here
Between the ages of 18-65
Primary diagnosis of schizophrenia with an acute exacerbation within the last 2 months.
If they were already inpatient, hospitalization must have started within 2 weeks of enrollment.
PANSS score between 80-120 (inclusive), and a score ≥48 on at least two of 4 selected positive symptom items9
CGI-S score needed to be at least a 4 (i.e. moderately ill)
Off all psychoactive medications for at least 1 week or 5 half-lives, whichever was longer
For patients previously on a LAI, most recent injection must have been >12 weeks prior
Frankly, the washout period is a bit short for my liking. Drug elimination half-life has a lot of individual variation. Take olanzapine for example; the range between the 5th and 95th percentile is 21-54 hours. That means that the 5th percentile only requires 4.4 days for complete elimination, while the 95th percentile requires 11.3 days. That’s not a small difference!
It’s not even clear from the study what authority they’re using to define a drug’s half-life, or if they’re considering active metabolites. Cariprazine has an active metabolite whose half-life is ~2 weeks; a full washout would take 2.5 months!
I don’t think this seriously undermines the results or anything, but it does make me wonder if the results would be substantially different in the treatment naive.
Exclusion Criteria
Individuals were excluded if:
They had a primary psychiatric disorder other than schizophrenia within 12 months prior to screening.
New diagnosis of schizophrenia OR no history of treatment for schizophrenia
History of treatment resistance to antipsychotic medication
Their PANSS score improved by more than 20% between screening and baseline (i.e. start of study)
Seems fine? It is worth nothing that the second bullet in the list means that this study is semi-enriched for previous responders to antipsychotics. I say “semi-enriched” because the criteria allows individuals who have tried and failed a single antipsychotic to thread the needle and be eligible for inclusion.
Along these lines, it’s also worth noting that patients did not have to be on treatment at the time of enrollment, and there was no timeframe in which they needed to have received treatment prior to enrollment. I’d want to know whether the randomization produced groups with statistically similar numbers of patients who had a remote history of treatment in both groups.
Interventions
Dosing started with xanomeline/trospium 50/20mg BID for 2 days, then 100/20mg BID for days 3-7. Starting on day 8, patients were increased to 125/30mg BID unless they were experiencing side-effects attributed to the drug. Patients could return to 100/20mg if they could not tolerate 125/30mg, but they could not return to 125/30mg afterwards. Dose reductions could not be made in the final 2 weeks of the study.
This seems well designed and prevents the sort of situation where the dosage range covers 4 commonly prescribed strengths and then the authors shrug their shoulders at you when you ask which one was most effective.
Patients were required to remain in an inpatient setting for the duration of treatment, and — as far as I can tell — they would’ve received care as usual on these units. There is no indication that either group received special attention or interventions beyond the check-ins for assessment on various metrics and side-effect screening.
Additional medications were not permitted without prior approval of the investigator, except in “medical emergencies.” Up to 6mg of lorazepam equivalents were permitted as prns for anxiety, agitation, and insomnia; z-drugs were also permitted as prn sleep aids.
The fact that these patients needed to be kept on an inpatient unit for at least 5 weeks is pretty rough, and makes you wonder how often agitation events resulted in IM antipsychotic administration in “medical emergencies.” They did keep records for these sorts of things, but they did not make the data available in any of the supplements, so we just have to guess. There is a lot of incentive to not use antidopaminergics (because it would make the placebo effect much bigger) so we’ll just have to trust that the investigators thought about this and avoided using them in substantial quantities.
Demographics
It’s a short table so I’ll just put it in here:
Baseline PANSS scores are ~97, which means most of the participants are at least what the CGI-S would call “markedly ill.” Otherwise nothing particularly interesting to comment on here.
Results
I’m just going to include the table from the paper’s supplemental so those interested in the top-line results can look them over first.
Overall PANSS Scores
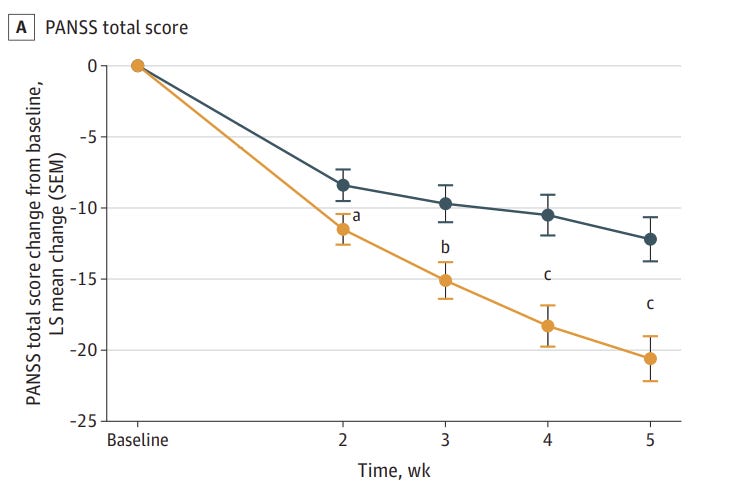
Xanomeline-trospium was associated with a statistically significant 8.4-point greater reduction from baseline to week 5 in PANSS total score compared with placebo; the LS mean (SE) change from baseline to week 5 in PANSS total score was −20.6 (1.6) in the xanomeline-trospium group compared with −12.2 (1.6) in the placebo group (LS mean difference, −8.4; 95% CI, −12.4 to −4.3; P < .001; Cohen d effect size, 0.60)
Before I start commenting, I want to put a big disclaimer up here. THE ONLY DATA WE HAVE GOES OUT TO 5 WEEKS - MAYBE THINGS LOOK BETTER AFTER 5 WEEKS. MAYBE THEY LOOK WORSE. PLEASE DO NOT WRITE ME ANGRY EMAILS TELLING ME THAT I AM A PHARMA SHILL/TOO MEAN TO BIG PHARMA BECAUSE I DIDN’T ACCOUNT FOR THIS. Thank you.
That’s A Nice Placebo Effect You’ve Got There
First, that’s a big placebo effect size! I thought that maybe this was some sort of issue unique to this study, but it turns out that antipsychotic effect sizes have been staying the same while placebo effect sizes have been creeping up. People in the field have been aware of this for at least a 15 years and it makes perfect sense that they would be. If drug effect sizes have been staying roughly the same, but placebo effect sizes keep creeping up, that poses a big problem when you try and convince the FDA to let you bring new treatments to market!
Stefan Leucht10 — who else? — has a paper discussing this very issue for the antipsychotics, though it’s also an issue across all of psychiatry. They found that for every 10 years between the publishing date of two papers, placebo response increases by 2.74 points on average. They find (and speculate on) a lot of reasons for this increase: increase number of study sites; use of the PANSS instead of the BPRS; shorter washout periods — the list goes on, though none of them account for more than ~10% of the heterogeneity observed.
Onset Of Effect is Comparable to the Antidopaminergics
If you look at that figure above, you see that xanomeline/trospium statistically separates from placebo at the 2 week mark, and that the improvement in PANSS scores are a little more than half of what they are at week 5.
This is pretty similar to the antidopaminergics, where >50% of all improvement is seen in the first 2 weeks. Does this suggest some final common pathway that can only change so fast?
A Good Effect Size?
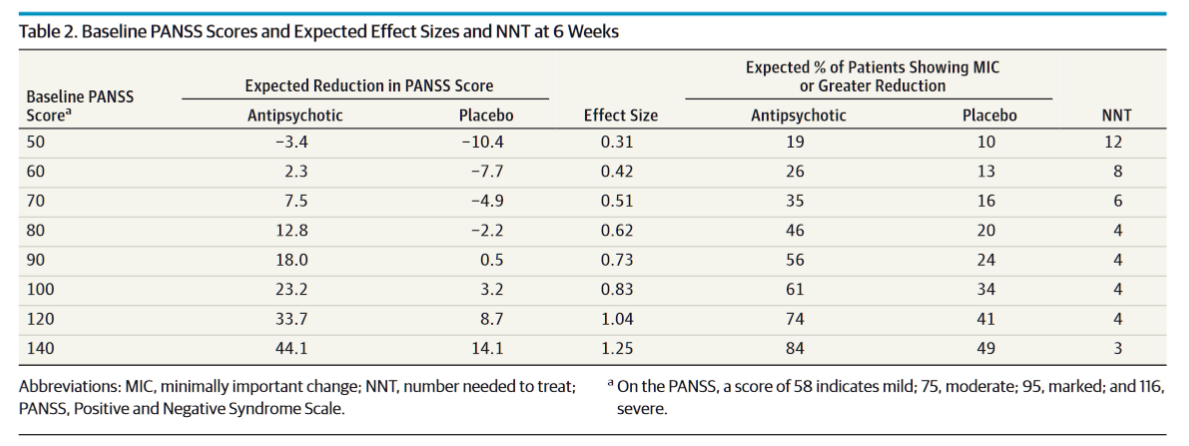
On the surface, an effect size11 of 0.60 for xanomeline looks pretty good. If you look at the big meta-analysis from Huhn et al. in 2019 that puts it solidly near the top of the list:
Now, this is just one study, n=256, nowhere near the amount of data we have for e.g. olanzapine and risperidone, but you’ve got to imagine that the investigators are pretty happy with that.
Except… in part 2 of my essay about picking antipsychotics and antidepressants, I pointed out that baseline PANSS scores have been shown to modulate the effect size of antidopaminergics. Specifically, antidopaminergics show a larger effect size the higher the baseline PANSS. Here’s the table I used from Furukawa et al.
Just to refresh your memory here, the average PANSS for this study was ~97 in all groups, and xanomeline reduced scores by 8.4 points with an effect size of 0.60. From this vantage, xanomeline actually underperforms the antidopaminergics by a decent amount at this PANSS score.
Is it a clinically relevant amount? I’m tempted to think so. You’d expect a 20 point reduction in PANSS scores with an antidopaminergic; 11.5 points better than xanomeline did here.
However, if you just look at the positive symptom subscore improvement, xanomeline performs the same as the antidopaminergics (d = 0.80 vs. d = 0.83) based on a baseline PANSS of 100. So where does it look like the discrepancy comes from?
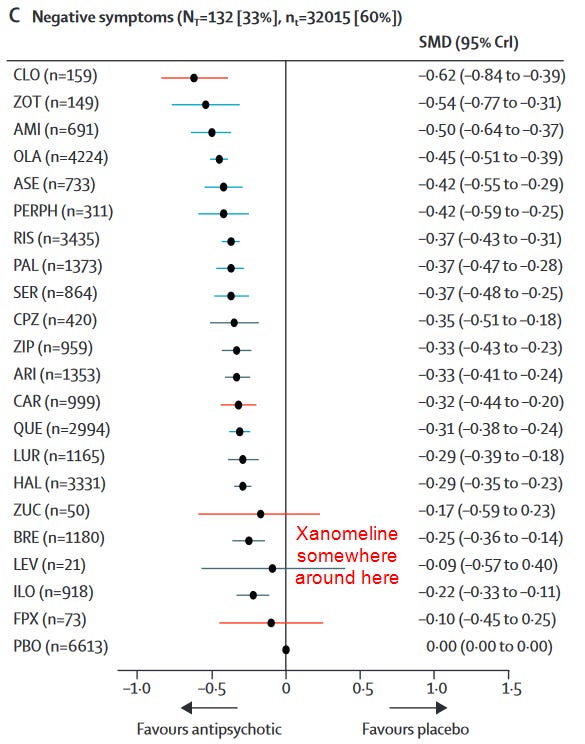
Negative Symptoms and Cognition, Surprisingly
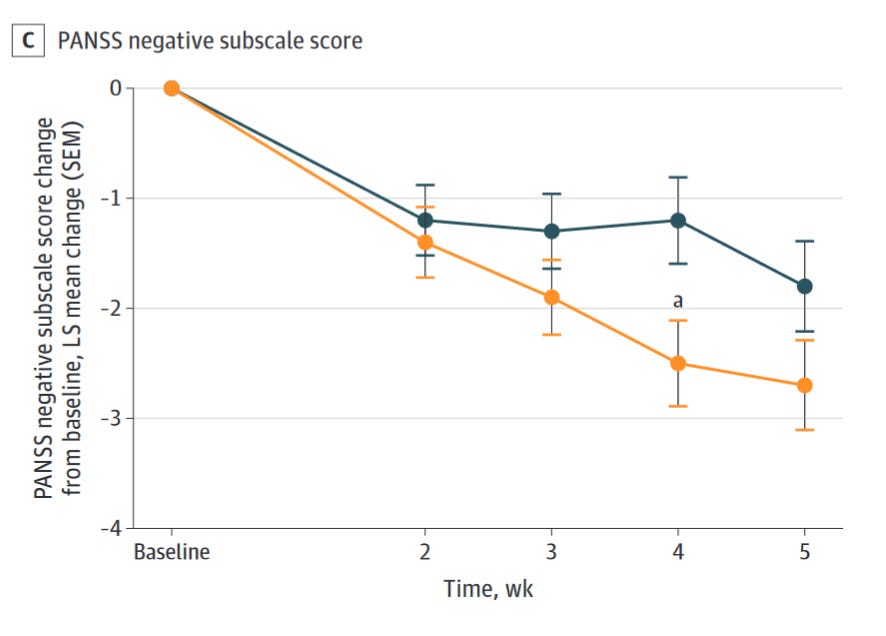
There’s something of a myth that the antidopaminergics don’t treat the negative symptoms of schizophrenia at all, when in reality their effect sizes are just more modest relative to improvements in positive symptoms. In any case, given the previous improvements in cognition seen with xanomeline, there was hope that it might improve the negative symptoms of schizophrenia and/or show cognitive benefits not seen with the antidopaminergics.
In this study, xanomeline briefly separated from placebo in negative subscores at week 4, but this significance disappeared at week 5. Xanomeline reduced PANSS negative scores by a mere -0.8 points (95% CI -1.9 to 0.2, P = 0.12; Cohen’s d = 0.23) relative to placebo.
There’s maybe something there, though even if we assume it’s real, a less than 2 point improvement isn’t exactly impressive. How does it stack up against the antidopaminergics as a whole?
Even if we throw xanomeline a bone and assume that with further studies this effect size separates from placebo, it is actually worse than most antipsychotics and markedly so in some cases. Xanomeline’s effect size is only half that of olanzapine’s, for example.
Cognition was an exploratory endpoint — along with change in baseline prolactin, response based on SNP schizophrenia subtypes and SNPs related to KarXT metabolism. They performed an automated neuropsych battery at baseline, Day 21, and Day 32 of the study. None of the exploratory endpoints were reported so I can’t draw any firm conclusions about why there’s no cognitive data, though you’d think it’s the sort of thing that they would have included in this paper if the readouts were positive.
Side Effects
The antidopaminergic antipsychotics have a lot of side-effects. Some, like cognitive blunting, can exacerbate some of the worse symptoms of the disease itself. Others, like tardive dyskinesia can leave patients stuck with very noticeable, involuntary facial tics for the rest of their lives, even if they stop taking the medication! More mundane side-effects include things like: olanzapine increasing your risk of developing diabetes by 3x; gaining lots of weight; orthostatic hypotension; sedation… the list goes on.
Xanomeline is not like this at all. Amazingly, its side-effects in this study were mostly limited to:
Mild/moderate gastrointestinal side-effects within the first 2 weeks of treatment that disappear with time.
Very small increases in BP (2-3mmHg) that peaked after 1 week of treatment
An increase in supine heart rate of ~10-13 bpm that decreased with time
No akathisia, no acute dystonia, no weight gain, no QTc prolongation (which doesn’t matter anyway). They also didn’t see any Parkinsonian symptoms or TD, and even though both generally take longer than 5 weeks in the antidopaminergics, the lack of dystonia and akathisia suggest that xanomeline isn’t screwing with nigrostriatal dopamine transmission very much.
If you’re wondering how much work the word “mostly” is doing before that list above… not much actually. I’ll let you look at the numbers yourself and decide:
Final Thoughts and Unanswered Questions
Xanomeline/Trospium (Cobenfy) is a new oral medication for the treatment of schizophrenia. It’s dosed BID in strengths of 100/20mg or 125/30mg and should be available starting sometime in October of 2024.
At five weeks it looks to be modestly less effective in reducing overall PANSS scores relative to the antidopaminergic antipsychotics — d = 0.60 vs. d = 0.83 at a baseline PANSS of 100. It performs comparably when looking at just the positive subscores, but it seems to be significantly worse at improving negative subscores relative to best-in-class antidopaminergics like clozapine and olanzapine.
As far as I’m concerned, xanomeline’s side-effect profile is where it stands out. Assuming there aren’t any nasty surprises waiting for us at the end of the long-term trials or distinct sub-populations with markedly different responses, I wouldn’t be surprised to see it supplant the SGAs as first-line for schizophrenia in the same way that the SRIs/SNRIs replaced the TCAs and MAOIs in all but the sickest patients.
That said, there are a lot of unanswered questions that we will need to see to further clarify xanomeline/trospium’s clinical role.
The biggest is of course what are its effects in the long term (i.e. past 5 weeks)? You probably noticed that in the graphs above, improvements in PANSS scores in the treatment group did not appear to have hit a plateau — you can see this quite well in the graph for the positive subscale:
The slope has just barely started to flatten out, which I think is quite promising! The good news is that there are already two 52 week open-label trials (EMERGENT-4 and EMERGENT-5) that are underway to study this question in an outpatient setting.
Some other interesting and important questions that this study did not answer:
Does xanomeline provide additional benefit as an adjunct to antidopaminergics, and vice versa?
Do “treatment resistant” schizophrenics respond to xanomeline?
Are there meaningful cognitive improvements?
Does xanomeline treat psychosis in other contexts (e.g. mood disorders)?
How effective is it in first episode psychosis and/or the treatment naive?
We’ll have to wait and see how this all shakes out. Exciting!
I had previously learned that the M4 agonism was why clozapine causes drooling, but the M1 agonism from norclozapine could also explain it!
Yeah, yeah I know that lockpicks for conventional locks are actually very simple, but I maintain that it’s much easier to come up with blocking a keyhole by e.g. shoving some clay into it, than it is to understand the mechanism of a lock well enough that you know you can just bypass it with a tension wrench and a rake.
That is, at a site that is distinct from where the endogenous ligand (acetylcholine) binds
Usually allosteric binding is modulatory (i.e. it does not directly activate the receptor itself), so this is interesting!
I am somewhat perplexed that this M5 antagonism isn’t considered to be part of the mechanism of action for xanomeline since M5 is mostly expressed in the dopamine rich regions of the midbrain!
Confusingly, a lot of papers about xanomeline also use the term “functional selectivity” in the sense that xanomeline binds all of the muscarinic receptors, but only activates M1 and M4.
For those wondering, the Ukrainian sites stopped enrolling new patients when the Russians launched their offensive in February 2022. Слава Україні!
At least moderately severe
Delusions, conceptual disorganization, hallucinatory behavior, suspiciousness/persecution
Whose last name is German for “light” - nominative determinism strikes again!
aka Cohen’s d







Why a placebo-controlled trial? I don't care how a new medication for schizophrenia compares vs placebo. I care how it compares vs standard of care - antidopaminergics. Placebo controlled trials are fair for conditions that don't yet have a standard of care. I want to see a trial where efficacy and tolerability are compared vs. a popular antidopaminergic (vs. combo?).
Good stuff. I haven't followed the KarXT stuff much so this is a good update. When I got to the end I was thinking what you were thinking - what about combination treatment? I'd be interested to see what xanomeline/trospium + really low dose haloperidol (or other high-potency 1st gen) does, as well as xanomeline/trospium + various SGAs.