Wot's Uh... The Deal With Antimuscarinics and Antihistaminergics in Delirium?
The mystery of low-dose Quetiapine for Delirium
Welcome to another installment in my Wot’s Uh… The Deal1? series, where I try and look closely at (what I think are) pharmacological mysteries in psychiatry and see if I can come up with some more satisfactory conclusions.
This week I’m writing about delirium and its pharmacological management, with a particular emphasis on the antidopaminergics.
What’s Delirium?
Delirium (sometimes called acute encephalopathy if you’re a neurologist2) is a complex syndrome that is nicely summarized by Wilson et al.’s 2020 review in Nature:
Delirium is a severe neuropsychiatric syndrome characterized by the acute onset of deficits in attention and other aspects of cognition. Patients often have altered arousal, from reduced responsiveness at a near-coma level to hypervigilance and severe agitation. They may also experience highly distressing symptoms of psychosis, including delusions and hallucinations, and altered mood. The features of delirium tend to fluctuate in presence and severity.
The DSM-5 defines it as:
A disturbance in attention, awareness, and one additional cognitive domain
That develops in a short period of time and tends to fluctuate over the course of a day
With evidence that it is caused by a medical condition, substance intoxication or withdrawal, or medication side effect.
I know what you’re thinking: “Finally, a clear and narrowly defined psychiatric illness!”
As you can imagine, things like severe agitation, hallucinations, disorientation, etc. etc. are not helpful in a hospital setting. They are especially unhelpful in when they cause critically ill patients - who have various IVs, drains, tubes, and so on that really really need to stay right where they are - to have strong disagreement with the USSR’s decision to strap them down to a bed and sap and impurify their precious bodily fluids for no good reason.
Delirium can happen to anyone, but there are some specific risk factors that make it more likely: pre-existing dementia, advanced age, low IQ, depression, substance use disorders, certain pre-existing conditions3, and visual/hearing impairments.
Most of these risk factors map well onto the idea of a low “cognitive reserve” - though not visual/hearing impairments4. Basically, something (or a collection of somethings) pushes your brain past its ability to keep things running smoothly - particularly its ability to maintain normal levels of attention and consciousness - and now brain no work good anymore.
There are many sophisticated hypotheses about the underlying biological causes of delirium that involve metabolic insufficiency, inflammatory changes, and the direct action of drugs/medications, which probably lead to to neuronal dysfunction, neurotransmitter derangements, and brain network disruption5.
If you’re still not clear, here is a “basic pathoetiological model of delirium.”

Don’t Worry! Psychiatry is Here To Er… At Least Not Make Things Worse? Probably?
Psychiatrists are often involved in the management of delirium in a hospital setting, though I’m not quite sure how we got involved, since it feels much more like a neurology thing than a psychiatry thing (I feel the same about sleep medicine). It may be because the current drugs of choice for treating delirium are mostly psychiatric medications, usually the antidopaminergics, which is really what I want to talk about.
The thing is… antidopaminergics don’t work for delirium.
Well, let me be more specific.
A 2018 Cochrane review of the use of antipsychotics in the treatment of delirium in non-ICU patients found that antidopaminergics:
did not reduce delirium severity… There was no evidence antipsychotics resolved delirium symptoms… The pooled results indicated that antipsychotics did not alter mortality…
It is worth noting, however, what this review was unable to comment on (emphasis mine):
No trial reported on duration of delirium. No trial reported on hospital length of stay, hospital discharge disposition, or health‐related quality of life. Adverse event reporting was limited and measured with inconsistent methods; in those reporting events, the number of events were low. No trial reported on physical restraint use, long‐term cognitive outcomes, cerebrovascular events, or QTc prolongation6…
A 2016 meta-analysis in ICU patients found that:
…Antipsychotic use was not associated with change in delirium duration, severity, hospital or ICU LOS, with high heterogeneity among studies. No association with mortality was detected
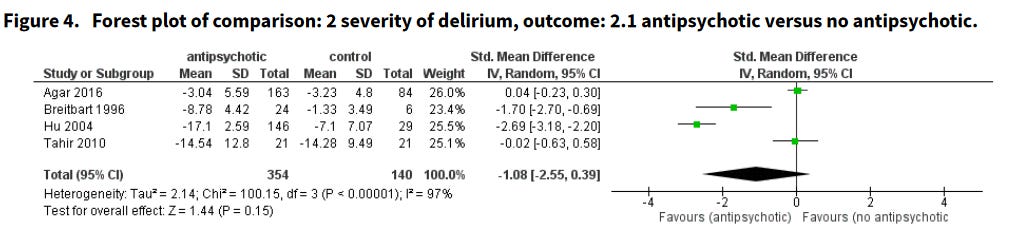
Importantly, these analyses indicate that antidopaminergics don’t appear to make delirium worse, and if you were going to bet on some effect, the graph below should update us slightly in favor of a positive one.
Still, these are null results. And yet — in every hospital I’ve worked in — haloperidol, quetiapine7, and olanzapine8 are usually the first-line treatments for agitated delirium. What’s going on here?
In Which We Find Our Hands Tied In An Attempt To Avoid Tying Theirs
I think the most reasonable argument I’ve run into as to why we should keep using the antidopaminergics is because it’s not like we actually have many other choices for managing acute agitation in the context of delirium.
And it’s true! Other than physically restraining patients9 - which everyone hates having to do - other pharmacological options are generally limited to:
Alpha-2 Agonists (dexmedetomidine10 and clonidine): Until recently, dexmedetomidine was basically never seen outside of ICUs because it was given as an IV drip and required close BP monitoring, but now has a sublingual formulation indicated for acute agitation.
Valproate11: There is some evidence for its use, but no RCTs. Usefully, it comes in lots of different formulations (IV, liquid, sprinkles12). Not so usefully, it can cause hyperammonemia and liver dysfunction.
Melatonin/Ramelteon: The literature here is quite mixed, but what’s new? A brief summary since I think the literature here is actually more fleshed out than usual (NB: I did not carefully read through all of these studies):
This 2023 meta-analysis from Aiello et al. looked at 9 RCTs that compared melatonin or ramelteon vs. placebo for delirium prevention in ICU patients (n=1625). In their initial analysis they did not find a difference in delirium incidence (RR 0.76 (0.54-1.07), p = 0.12; I2 = 64%), but they did in their sensitivity analysis that included 4 other non-RCT studies (RR = 0.67 (0.48-0.92), p=0.01; I2 = 67%). There was no difference in duration of ICU stay, duration of mechanical ventilation, or mortality.
A 2021 review from Khang and Nair looked at 14 RCTs (only 3 of which overlapped with the Aiello review?!) that compared melatonin or ramelteon vs. placebo for delirium prevention in hospitalized patients (n=1712). They found a significant decrease in delirium incidence overall (RR 0.61 (0.42–0.89), p=0.009), but subgroup analysis showed this was only significant in ICU and surgical patients and did not extend to medical patients. Heterogeneity was quite high (I2 = 66%), but they did do a fixed-effects model to correct for this and still found a statistically significant result (RR 0.7 (0.59-0.84), p<0.0001). The biggest blemish here is a funnel plot that indicates significant publication bias. It also could not include the massive (n=847) Wibrow et al. study from 2022 that found no effect.Dual Orexin Receptor Antagonists (aka DORAs: suvorexant, lemborexant) - There are actually quite a few RCTs and retrospective case-control studies, in ICU, surgical, and general medical patients, all out of Japan. Xu et al.13 analyzes 7 of these (four RCTs, three retrospective) and finds that delirium incidence was reduced significantly (OR 0.3 (0.21-0.44), p<0.001, I2 = 24%). Though, as usual, there was no reduction in hospital or ICU length of stay, time on ventilation, or mortality.
“What about the benzodiazepines?”
Is actually a pretty reasonable question if you’re not a psychiatrist. They’re sedating and anxiolytic, we give them to people who are psychotic and agitated, it’s a pretty logical idea.
Doesn’t turn out to be a good idea though.
There is decent evidence that benzodiazepines increase the risk of developing delirium in geriatric populations. This systematic review by Clegg and Young from 2011 collects seven studies that look at benzodiazepine use and all of the ones I looked at found that benzodiazepines either resulted in higher rates of delirium or a longer duration14.
However, RCTs on this question are scant and those that do exist do not clearly show higher risks for benzodiazepines. Take this meta-analysis by Fraser et al. from 2013 that compared benzodiazepines and dexmedetomidine. It found a shorter length of ICU stay and duration of ventilation for dexmedetomidine, but no difference in the prevalence of delirium between groups.
Almost all of this data is in geriatric populations, so it’s unclear if benzodiazepines might be more useful/less harmful in younger individuals. That said, I think the majority of the data here points in the same direction and tends to match up with clinical experience, to the point that it seems unwise to use benzos unless you have a very strong clinical rationale (e.g. you think this is delirium tremens; palliative care settings)
So, No Anticholinergics/Antihistamines, Right?
Yes, good job.
Anticholinergic medications - which we should all just call antimuscarinics because that’s what they actually are (I’m starting now) - have been shown to induce generalized slowing on EEGs and changes in delta wave activity that are associated with altered arousal and reduced cognitive ability.
A 1999 paper from Tune and Egeli used a radioreceptor assay to compare the level of antimuscarinic activity in serum between patients. They found that patients with high serum antimuscarinic activity and those that are on multiple antimuscarinic medications were more likely to become delirious. Additionally, they cite other studies that showed elevated serum antimuscarinic activity in delirious individuals relative to non-delirious patients, and a reduction in antimuscarinic activity when delirium resolved15.
Similarly, the antihistamines16 are well known to cause sleepiness and reduced reaction time; in overdose, they produce states that absolutely sound like delirium (just go read the /r/DPH subreddit). We also have a decent understanding of the neurobiological role that histamine plays in wakefulness. This paper from Chazot has a concise overview.
The Conclusion
So yeah, maybe give the DORAs and melatonin/ramelton a shot. Antidopaminergics are questionable and but don’t seem to make things worse. Don’t use benzodiazepines. Don’t use anti-muscarinic or anti-histaminergic medications. Definitely don’t use medications that antagonize both. Can you imagine how bad and irresponsible that would be? Good thing we would never, ever do that.
What’s that?
Oh, a screenshot from a Wikipedia article? You can just leave it down below, I’m done with this essay anyway. Nice to be able to wrap this one up succinctly.
Yeah, but like olanzapine is pretty potent at D2 so that probably expl-
You have another? It’s not, uh, that one, is it?
…shit
The Mystery of Antipsychotics in Delirium
Are you confused? I’m confused. If you’re not confused, you should be confused. None of this makes any sense. The fact that antidopaminergics don’t improve delirium speaks against a hyper-dopaminergic hypothesis, but then why don’t quetiapine and olanzapine — two medications that are highly antimuscarinic and antihistaminergic — just make everything worse?
“Well, maybe the dopamine antagonism-”
Yeah, let me stop you right there.
We Need To Talk About Quetiapine
Let’s revisit the tables above, but this time simplified and with haloperidol and diphenhydramine included. In places where the ranges were much too wide17, I used the median from the human data in the PDSP Ki Database.
Just as a reminder, the lower the Ki, the higher affinity the drug has for a given receptor.
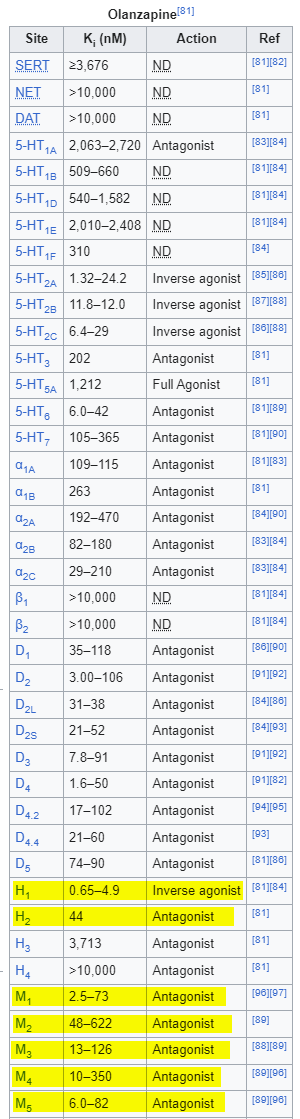
You might have noticed that quetiapine is very bad at antagonizing the D2 receptor. You know, the primary mechanism by which antipsychotics do their whole antipsychotic thing. In fact, quetiapine has the lowest affinity for D2 of all of the antipsychotics, which I’m sure it is very embarrassed about. On the other hand, quetiapine and its metabolite norquetiapine are very good at blockading H1 and M1 — even better than Benadryl! Something we would never consider giving to a delirious person. Ever.
What this means in practical terms is that quetiapine does not actually cause D2 blockade below doses of 450mg/day (there are multiple studies on this). At doses north of 150mg, quetiapine develops its “antidepressant” effects, which has been erroneously attributed to norepinephrine reuptake inhibition, and might be better explained by alpha-2c antagonism?18 It’s very unclear. Functionally, this means that at low doses of quetiapine — let’s say doses <150mg — it’s basically just an antagonist at H1, M1, alpha-1a, and alpha-2c.
In any case, quetiapine antagonizes everything that you’re not supposed to in delirium! The alpha-2 antagonism is particularly strange considering that dexmedetomidine works by being a alpha-2 agonist.
And yet there are multiple papers that use low doses of quetiapine and keep finding that not only does quetiapine not make delirium worse, but might actually improve some features of the illness.
Take this double-blind RCT from Kim et al. 2020 (n = 37) which showed that very low dose quetiapine (12.5mg to 25mg qHS) as a prophylactic reduced delirium duration and improved rates of weaning from mechanical ventilation.
Or another double-blind RCT from Devlin et al. 2010 (n=36) which showed that patients with delirium, on an average dose of 110mg/day (IQR: 88-191mg) had a reduced duration of delirium and less agitation.
Wait, Isn’t Olanzapine A Problem Too?
Yeah, it’s a fair point. Olanzapine has much stronger M1 affinity than quetiapine/norquetiapine.
A key difference is that olanzapine’s D2 antagonism even at low doses is pretty substantial. This AJP study showed that patients on doses as low as 5mg/day had D2 occupancy between 43-64%, and reached >70% at 10mg and above.
What’s Going On Here?
No idea.
Here are some theories.
Option 1: We’re Making Things Worse Under the Hood
One thought might be that the H1 and M1 antagonism actually does make things worse, but the D2 antagonism masks this by suppressing psychomotor agitation and so it just looks like it’s working/at least not making things worse.
Unfortunately, this doesn’t make sense when you remember that quetiapine at low doses isn’t blocking D2 at all.
Additionally, olanzapine’s affinity for H1 and M1 is so high relative to D2 that you would expect there to be a period of time where significant D2 blockade ends but there is still significant H1 and M1 antagonism and people should look much worse. That doesn’t really seem to happen19, but I’m not sure that anyone has looked closely at this.
Option 1b: We’re Just Turning HYPERactive Delirium Into HYPOactive Delirium
Maybe H1/M1 antagonism doesn’t make things worse and just improves the stuff that everyone really cares about (e.g. attempting to protect their precious bodily fluids) via sedation.
Option 2: Quetiapine Does Different Things At H1?
As I was researching this essay, I came across an interesting quote from this article:
Constitutive GPCR activity is recognized for many GPCR family members and results in GPCR signaling without the need of an external agonist. This spontaneous GPCR signaling is thought to evolve from the conformational dynamics of GPCR proteins, resulting in equilibria between active and inactive receptor states… Agonists drive the equilibria toward active GPCR conformation(s), whereas so-called inverse agonists would favor the inactive conformations. Following this notion, many of the known GPCR antagonists (including the histamine receptor antagonists) have been reclassified as inverse agonists, whereas true (neutral) antagonists are difficult to identify for most GPCRs.
Assuming you have a photographic memory, you might recall that olanzapine acts as an inverse agonist at H1, while quetiapine is just called an “antagonist.” I think this probably just means that it is also an inverse agonist, but the people writing the Wikipedia article just didn’t know about the distinction at the time.
But since we don’t have a good explanation anyway, it’s worth at least considering that quetiapine might be a “pure” antagonist, and the intrinsic activity of the H1 receptors that quetiapine blocks allows it to be sedating without increasing delirium risk.
There are a bunch of other things that might be explanatory along these lines. Maybe quetiapine exhibits functional selectivity (i.e. effectively transforms one receptor type into another, like LSD does to 5HT2A). Maybe it causes weird receptor heterodimers to form.
Sometimes I wonder how we ever figured out anything about pharmacology at all.
Option 3: Occam’s Least Favorite
Maybe olanzapine and quetiapine both just happen to block the right combination of receptors that produces some sort of mildly-positive-and-at-least-not-harmful-effect in delirium? Maybe like how you might put together a combination of notes on a piano that sound sharp and grating individually, but together form a pleasing (if slightly unnerving) chord20.
I guess this could be correct, but then you’d have to explain why haloperidol — which doesn’t have any of the H1 or M1 activity — seems to do just as well as olanzapine and quetiapine in dealing with the symptoms of delirium.
Option 4: 5-HT2A Antagonism Is Important
Normally when we talk about 5HT2A antagonism with the antidopaminergics, it’s about whether or not there’s enough 5HT2A antagonism that exists to counteract the extrapyramidal side-effects caused by strong D2 antagonism in the nigrostriatal pathway.
But what if 5HT2A antagonism itself is helpful in delirium? Antagonism here is thought to contribute to the hypnotic effects of some medications. And if we look at the receptor affinities again…
We can see that olanzapine, quetiapine/norquetiapine, and haloperidol all have enough 5HT2A antagonism that we would expect to see some activity at doses used in delirium.
This is at least a little supported by the other literature on compounds with strong 5HT2A antagonism, though be mindful that these are all small studies and not RCTs.
Mohammadi et al. found that prophylactic cyproheptadine decreased the incidence (though not the severity) of post-operative delirium.
Nakamura et al. found that mianserin was just as effective as haloperidol in treating delirium.
Tanaka et al. also found a 60-70% response rate with mianserin21 in patients with delirium
Lastly (and least convincingly) is Okamoto et al. which purports to show rapid improvement in delirium with trazodone, though is perhaps more interesting because every one of the 9 patients in this study were also being treated with some sort of benzodiazepine!
Looking at how the Ki’s line up together:
And then alongside the antidopaminergics:
This actually looks like a pretty reasonable hypothesis! I think it’s made even stronger by the fact that mianserin and cyproheptadine have high affinity at H1. Trazodone doesn’t look as strong, but this paper indicates that even at doses of 50mg it’s blocking somewhere in the neighborhood of 80% of H1 receptors.
Those of you who are familiar with LSD and the other classic psychedelics like psilocybin and DMT may recall that their primary mechanism of action is 5HT2A agonism. Maybe antagonizing these receptors is helpful in preventing/lessening some of the hallucinatory symptoms? I’m just speculating here in this entire section.
Option 5: Antihistamines and Antimuscarinics Are Ok In Delirium Actually?
I think a closer reading of the literature at least gestures in this direction.
First, let's return to that Clegg & Young systematic review I mentioned above on regards to benzodiazepines. It doesn’t find a clearly increased risk of delirium with H1 antagonists (OR 1.8, 95% CI 0.7–4.5), though it does with benzodiazepines (3.0, 1.3–6.8) and opioids (2.5, 1.2–5.2).
Next, the story that 1999 Tune paper told about serum antimuscarinic activity (SAA) has gotten shakier with time.
van Munster et al. — a longitudinal study of 142 elderly patients admitted for hip fracture surgery — did find that SAA was higher in delirious vs. non-delirious patients, but that this difference disappeared when controlling for the timing of the operation.
Thomas et al. looked at 61 elderly patients see if SAA was associated with the classic EEG changes22 seen in delirium. They found that, “EEG measures correlated significantly with cognitive performance and delirium severity, but not with SAA levels.”
I think that this is the least-complicated and most-straightforward interpretation, and probably the one that is most likely to be missed because (like QTc) there is a mythology around how Bad and Irresponsible it would be to give these medications in delirium. It would also remove the need for any of the fancy hypothesis above.
What Does It All Mean?
Again, no idea. The more you think about this, the less sense it makes. The depressing bottom line is this:
We have no good evidence that our pharmacologic interventions for delirium actually do anything to:
Reduce the length of ICU or hospital stays
Reduce the duration of delirium
Reduce the severity of delirium
Reduce the time on a vent
On that cheerful note, some personal takeaways:
I still think benzos are risky business in delirium and should be avoided unless you think the patient is in GABAergic withdrawal.
I previously believed that antihistamines/antimuscarinics were very likely to increase the incidence of delirium and/or make delirium worse and should be avoided. I would have recommended discontinuing medications like low-dose quetiapine or diphenhydramine regardless of observed patient response. I am now much less confident about this and will adjust in the following ways:
I still prefer olanzapine or haloperidol as a first-line agent in agitated delirium because it seems likely that D2 antagonism is useful for psychomotor symptoms.
I would not consider using low-dose quetiapine (receptor affinities are too messy), but would consider using a more pure H1 antagonist like hydroxyzine.
I would no longer recommend discontinuing something like low-dose quetiapine if it shows good clinical effect.
I have updated away from melatonin being useful for delirium prevention, though it doesn’t seem harmful.
I will try and use the DORAs more often in delirious patients.
Trazodone and cyproheptadine will be added to my bag of speculative tricks for particularly severe cases of agitated delirium.
This is a Pink Floyd reference, by the way
There are arguments about the differences between the two, but from what I can tell the consensus is that they are pretty much the same thing.
Too numerous to list
I’m guessing this has something to do with both of these being important in providing information necessary for orientation?
Again, go read that Nature Review, it’s fantastic.
Though, I don’t think you should care about that and I wrote a whole article on why:
Seroquel
Zyprexa
Which comes with its own sets of risks (e.g. Chou et al. 2020)
Precedex
Depakote
An argument for including Abbot or Sanofi branded soft-serve ice-cream machines in the PACU and geri units, I think.
I think it’s worth highlighting that this is an all Chinese team publishing in a very low impact factor journal. This always sets alarm bells off in my head, especially after reading this excellent piece of investigative journalism from Science - though given that this is just an analysis of studies done elsewhere, I’m less concerned.
Intuitively, higher doses also increased risk for delirium. Perhaps not-as-intuitively, longer acting benzodiazepines also increased risk for delirium. This sounds logical, but I think it could’ve gone either way. You could argue that the short-duration changes in cognition caused by the short-acting benzos could be more disruptive/disorienting than the long-acting changes.
This is a footnote to foreshadow that this is not as clean as it seems.
i.e. centrally acting H1 and H2 antagonists. Think Benadryl/diphenhydramine.
e.g. Olanzapine’s D2 affinity ranging from 3-106 nM
See The Last Psychiatrist’s great breakdown as to why: https://thelastpsychiatrist.com/2010/02/how_seroquel_xr_works_part_1.html
And this could be because olanzapine’s half life is so long, ~45h in the elderly, that they’ve gotten dosed again before enough olanzapine has washed out to see this effect.
I am not sure if this is a good analogy from a music theory perspective or not, but I’m not exactly intimidated by pianists.
The way this article is written implies that the Japanese think that mianserin is pretty standard stuff for delirium.
e.g. Occipital slowing, reduced alpha wave amplitude, increased delta and theta wave amplitude.








Thank you for that! The anti-muscarinic paradox with Olanzapine and Quetiapine has bothered me for some time.
I find the 5HT2a hypothesis very compelling. It should be interesting to examine it via some mechanisms described in the paper by Nutt and Carhart-Harris "Serotonin and brain function: a tale of
two receptors" (https://journals.sagepub.com/doi/pdf/10.1177/0269881117725915) and through the lens of the entropic brain hypothesis - maybe the delirious brain is in a more entropic state which can be (kind of) specifically attenuated by 5HT2a antagonism?
I’d say your pick of diphenhydramine over quetiapine doesn’t compute for me, I also have questions about its efficacy in other disorders specifically how it is first like in Parkinson’s psychosis with little chemical “antipsychotic” effect. A mystery drug to be sure.